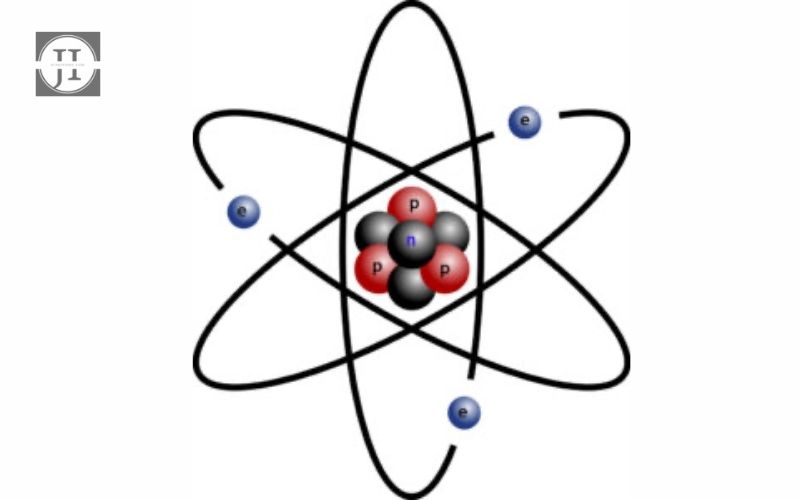
In the vast realm of science, the atom stands as the fundamental building block of matter, a mysterious entity that has captivated the minds of scientists for centuries. One pivotal figure in unraveling its secrets was James Chadwick, whose groundbreaking experiment in the early twentieth century revolutionized our understanding of atomic structure. In this blog post, we will delve into the intricacies of the atom, explore Chadwick’s experiment, and uncover the profound impact his discovery of the neutron had on the fields of physics and beyond. Prepare to embark on a journey through the fascinating world of atomic science.
Understanding The Atom
The study of atoms is a fundamental aspect of modern scientific research. Atoms are the smallest units of matter that retain the properties of an element, and they are composed of even smaller particles called protons, neutrons, and electrons. In order to understand the nature of atoms, scientists have conducted numerous experiments and made significant discoveries. One of the key advancements in this field was the discovery of the neutron, which played a crucial role in shaping our understanding of the atom.
The discovery of the neutron is credited to Sir James Chadwick, an English physicist, in the year 1932. Prior to this, scientists knew about the existence of protons and electrons, but the nature of atomic nuclei remained a mystery. Building on the work of his mentor, Ernest Rutherford, Chadwick performed a series of experiments that led to the identification of neutrons. His experiments involved bombarding different elements with high-energy particles to observe the resulting radiation. Through meticulous analysis and a keen eye, Chadwick successfully demonstrated the presence of an uncharged particle with a mass similar to that of a proton – the neutron.
James Chadwick’s Experiment
James Chadwick was a British physicist who is credited with discovering the neutron in 1932. At the time, the atomic model proposed by Ernest Rutherford consisted of a positively charged nucleus containing protons, surrounded by negatively charged electrons. However, this model was unable to explain the overall mass of an atom. In order to solve this puzzle, Chadwick conducted a series of experiments that eventually led to the identification of the neutron.
Chadwick’s experiment involved bombarding a thin sheet of beryllium with alpha particles, which are positively charged particles. He observed that as a result of this interaction, neutral radiation was emitted. By carefully measuring the energy and trajectory of this radiation, Chadwick was able to determine that it consisted of particles with approximately the same mass as a proton, but with no charge. He concluded that these particles were neutral and called them neutrons.
This monumental discovery filled a crucial gap in our understanding of atomic structure. Prior to Chadwick’s experiment, scientists were perplexed by the fact that the mass of an atom seemed to exceed the combined mass of its protons and electrons. The discovery of the neutron resolved this discrepancy, as it accounted for the additional mass.
Chadwick’s experiment revolutionized the field of nuclear physics and had a profound impact on various scientific disciplines. The identification of the neutron opened up new avenues for research and paved the way for the development of atomic energy. Scientists now had a more complete understanding of atomic nuclei, which enabled them to explore the possibility of nuclear reactions, leading to the harnessing of nuclear power.
The Impact Of The Neutron Discovery
The discovery of the neutron was a groundbreaking moment in the field of atomic physics. At the time, the structure of the atom was still not fully understood, and the existence of a neutral subatomic particle was only theorized. It was not until James Chadwick’s experiment in 1932 that the neutron was officially discovered. This discovery had a profound impact on our understanding of nuclear reactions and the stability of atomic nuclei.
One of the major impacts of the neutron discovery was the development of the atomic bomb. The ability to control and harness the power of atomic nuclei led to the creation of devastating weapons that forever changed the course of warfare. The discovery of the neutron provided crucial insights into nuclear fission, the process by which the nucleus of an atom splits into two smaller nuclei, releasing a huge amount of energy. This understanding paved the way for the development of nuclear weapons, which had a significant impact on global politics and military strategies.
Furthermore, the neutron discovery revolutionized the field of nuclear energy. By understanding the behavior of neutrons within atomic nuclei, scientists were able to develop the concept of controlled nuclear chain reactions. These chain reactions, which occur in nuclear power plants, produce a continuous release of energy that can be harnessed for electricity generation. The discovery of the neutron opened up new possibilities for sustainable and clean energy production, reducing reliance on fossil fuels and mitigating the impact of climate change.
Overall, the impact of the neutron discovery cannot be overstated. It fundamentally transformed our understanding of atomic structure and laid the foundation for the development of atomic weapons and nuclear energy. The discovery of the neutron paved the way for unprecedented scientific advancements and opened up new frontiers in both warfare and energy production. By unlocking the secrets of atomic nuclei, scientists have been able to tap into the immense power that lies within the atom, forever changing the course of human history.